PRIMARY EFFICACY & SAFETY
VILTEPSO significantly improved dystrophin production
Result Details
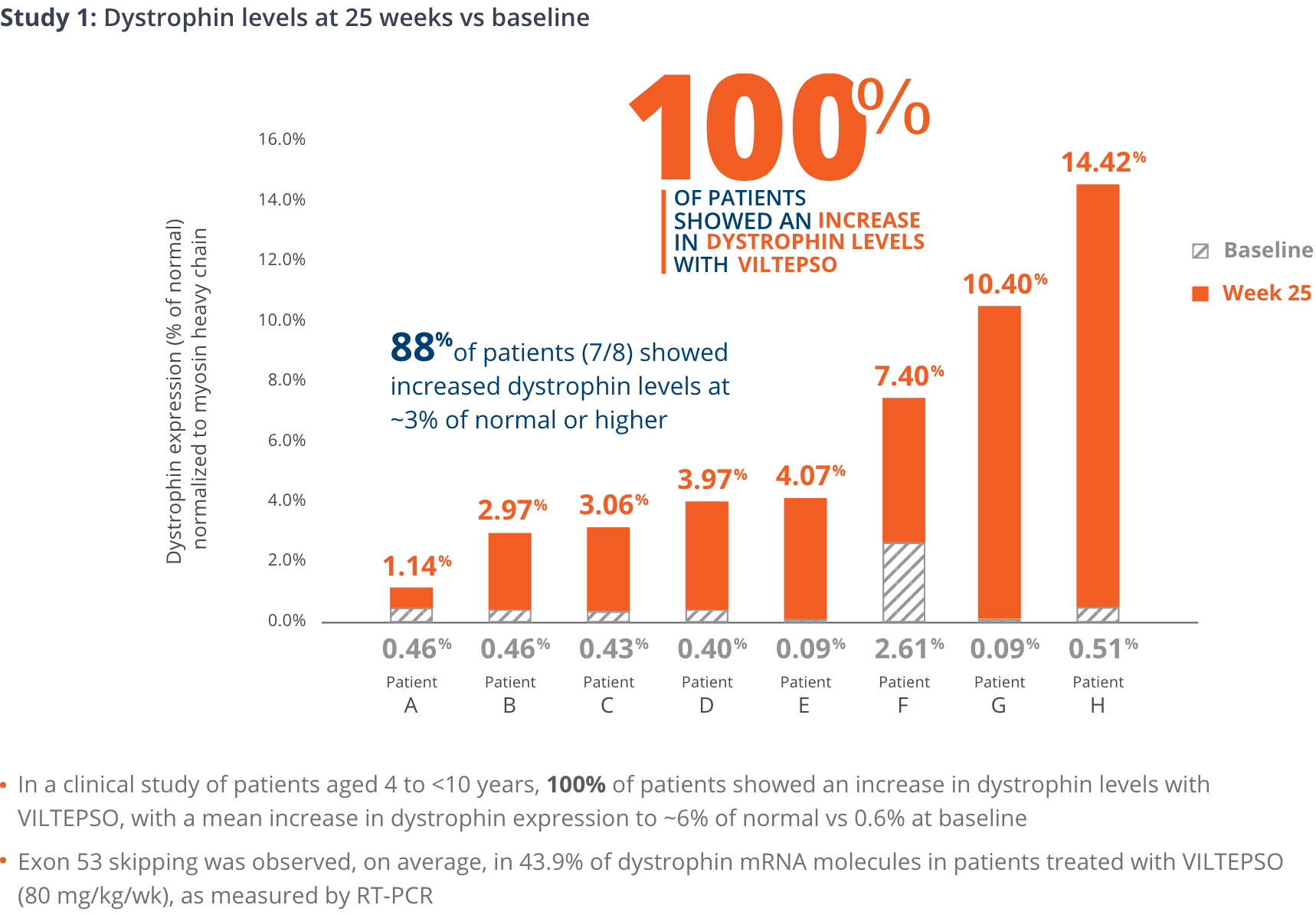
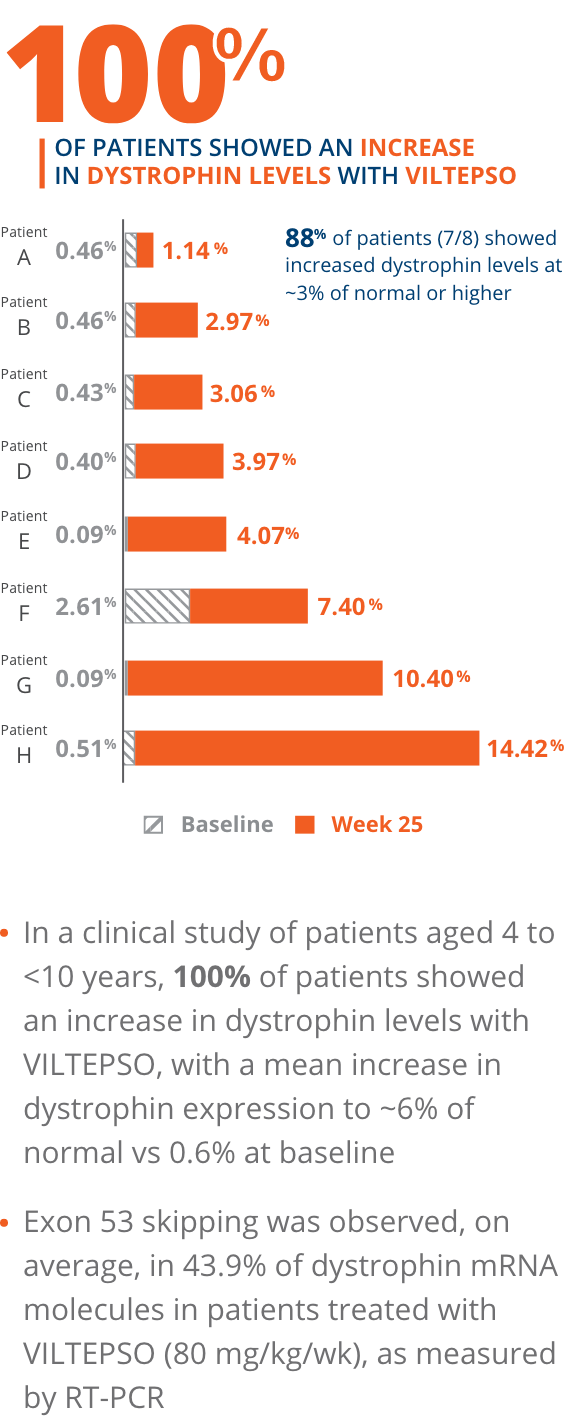
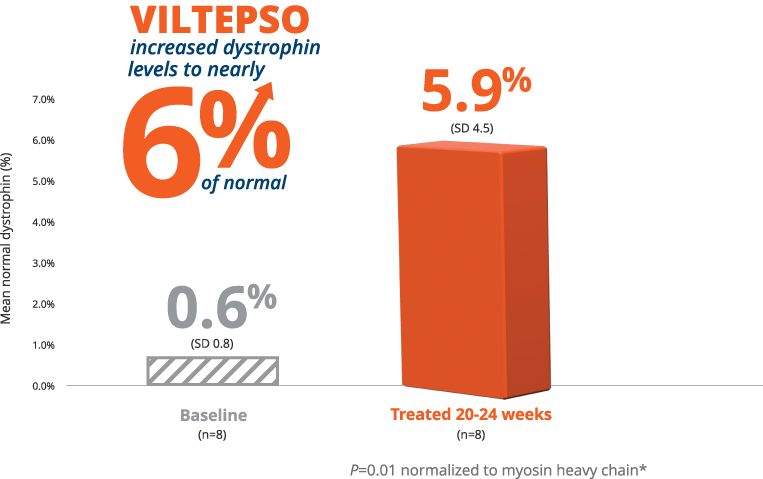
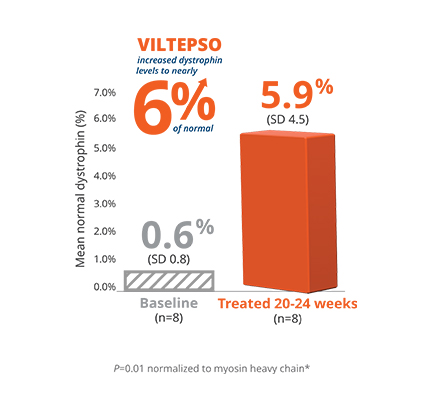
- Mean dystrophin levels increased to nearly 6% of normal with VILTEPSO (80 mg/kg/wk) vs 0.6% at baseline
- The statistically significant increase in dystrophin expression was measured by western blot analysis, a validated, highly sensitive, and reproducible methodology. Dystrophin protein level (normalized to myosin heavy chain) was based on the change from baseline, measured as percentage of the dystrophin level in healthy subjects at week 25
- Mean change in dystrophin was 5.3% (SD 4.5) of normal levels (p=0.01)
- Median change from baseline was 3.8%
*P-value for change from baseline at week 25 was statistically significant.
Study Design Details
Study design: A 2-period, randomized, double-blind, placebo-controlled, dose-finding study, with ambulant males aged 4 to <10 years with a confirmed mutation of the DMD gene amenable to exon 53 skipping who were receiving a stable dose of corticosteroids for ≥3 months (N=16). Patients received a once-weekly infusion of 40 or 80 mg/kg viltolarsen or placebo for a 4-week safety period followed by a 20-week open-label study to assess the efficacy and safety of viltolarsen. After completion of the 24-week study, patients could enroll in up to a 192-week extension study with efficacy assessments conducted every 12 weeks. Patients were required to remain on a stable dose of glucocorticoids for the duration of the study.
DYSTROPHIN WHERE IT MATTERS
VILTEPSO-induced increases in dystrophin levels were correctly localized to skeletal muscle cell membranes, as confirmed by immunofluorescence staining
100% of patients showed an increase in dystrophin levels with VILTEPSO
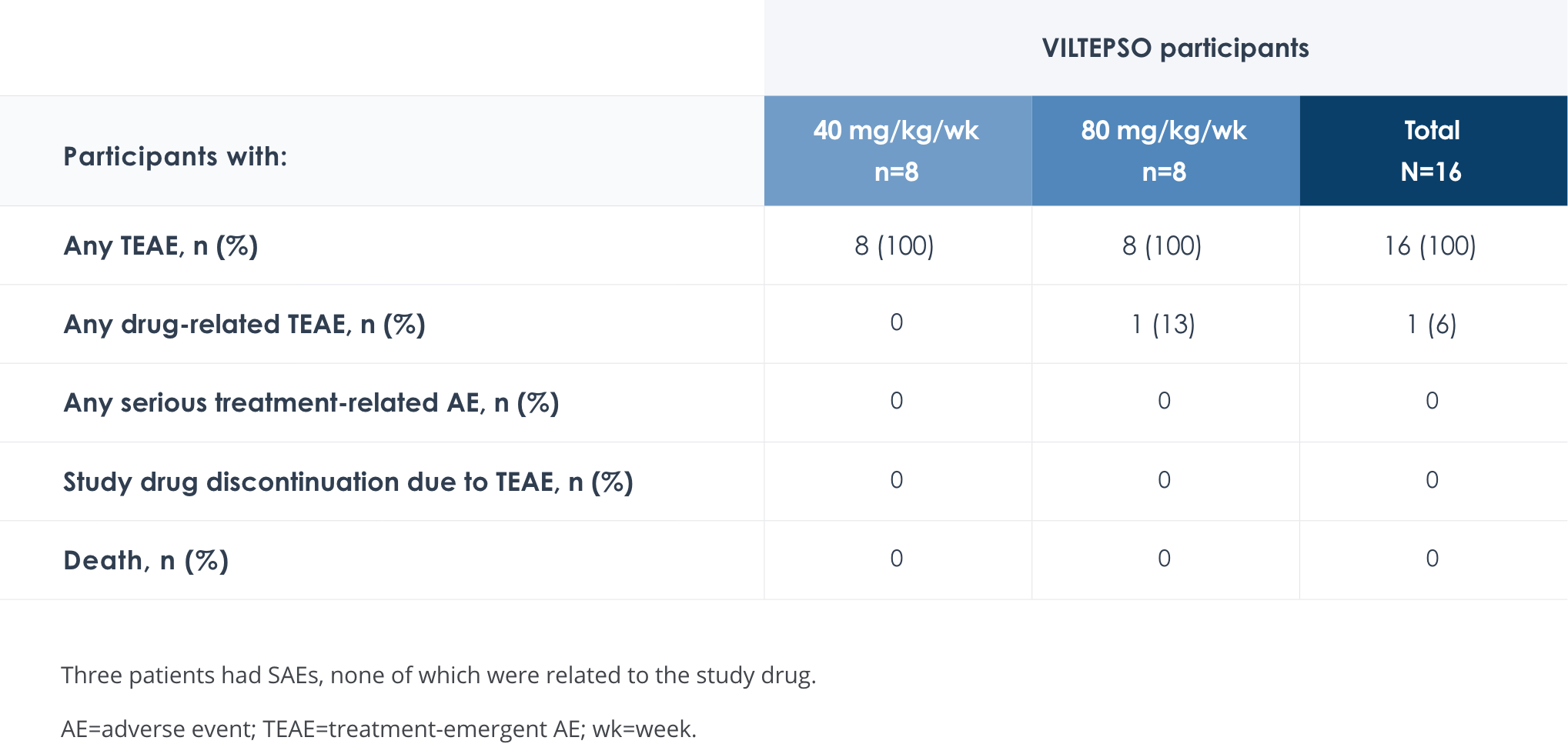
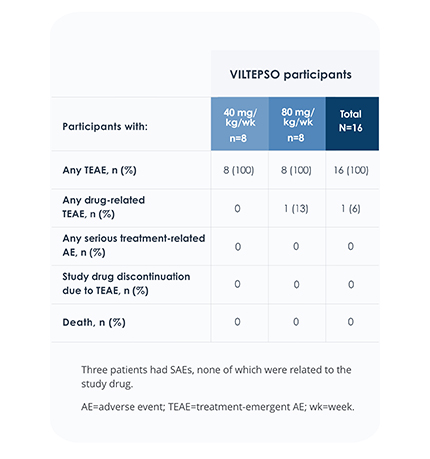
Safety of VILTEPSO was evaluated in two 24-week clinical studies
Adverse reactions reported in ≥10% of DMD patients treated with VILTEPSO 80 mg/kg once weekly
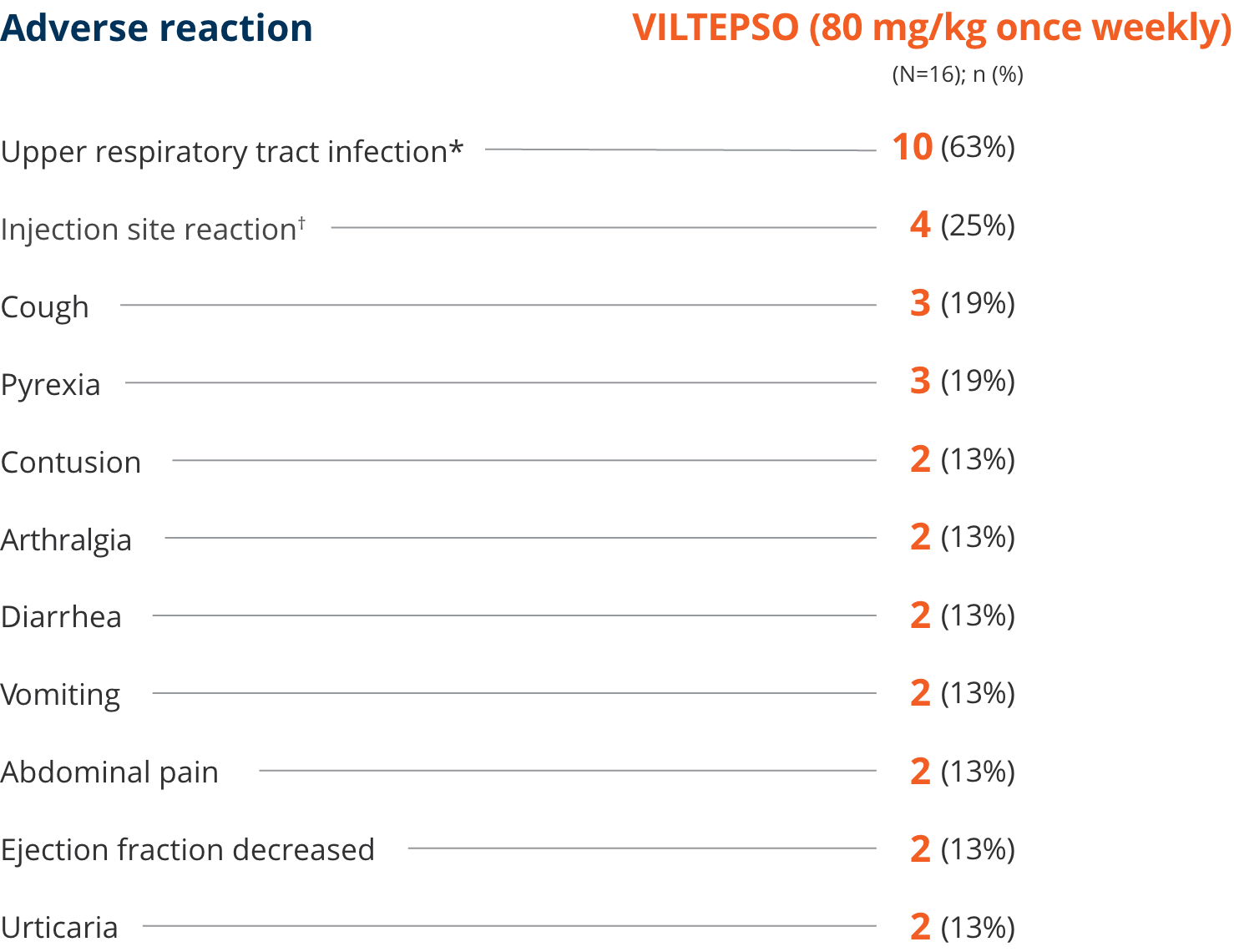
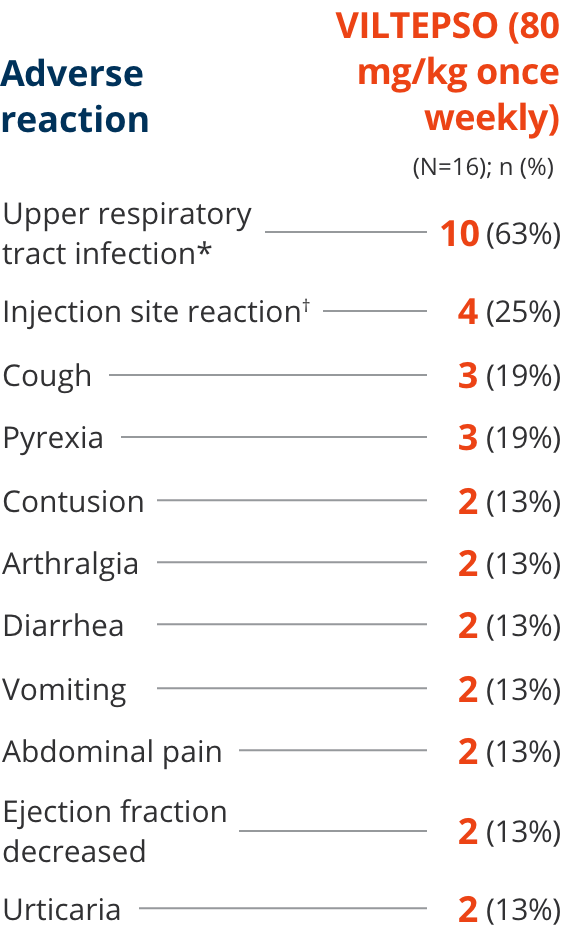
| * | Upper respiratory tract infection includes the following terms: upper respiratory tract infection, nasopharyngitis, and rhinorrhea. |
| † | Injection site reaction includes the following terms: injection site bruising, injection site erythema, injection site reaction, and injection site swelling. |
| This profile includes data from a multicenter, parallel-group, open-label, dose-finding study conducted in Japan in ambulant and non-ambulant males aged 5 to <18 years with a confirmed mutation of the DMD gene amenable to exon 53 skipping. Non-ambulant is defined as patients with a 6MWT distance of <75 meters. |
No treatment-related SAEs, drug-related TEAEs, discontinuations, or deaths occurred
SAE=serious adverse event;
TEAE=treatment-emergent adverse event.
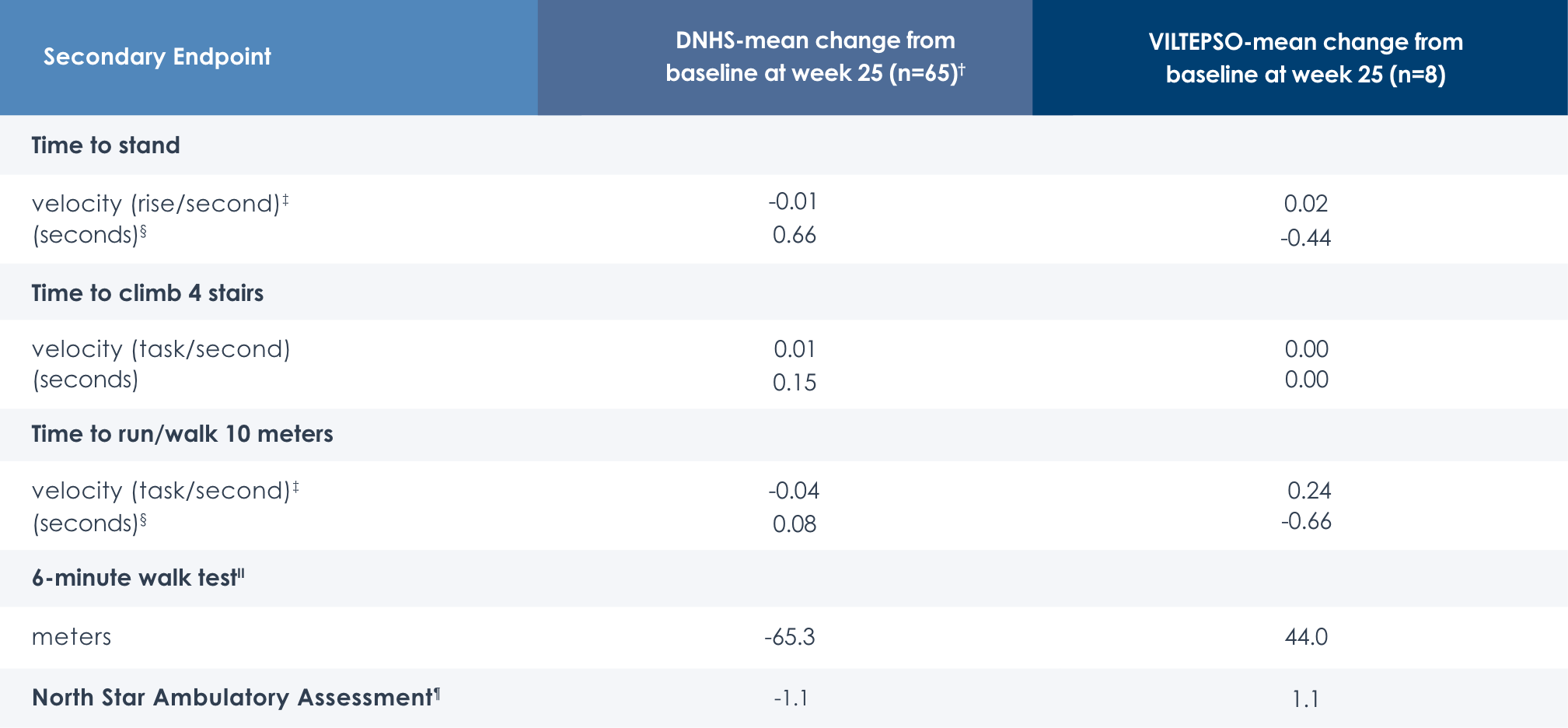
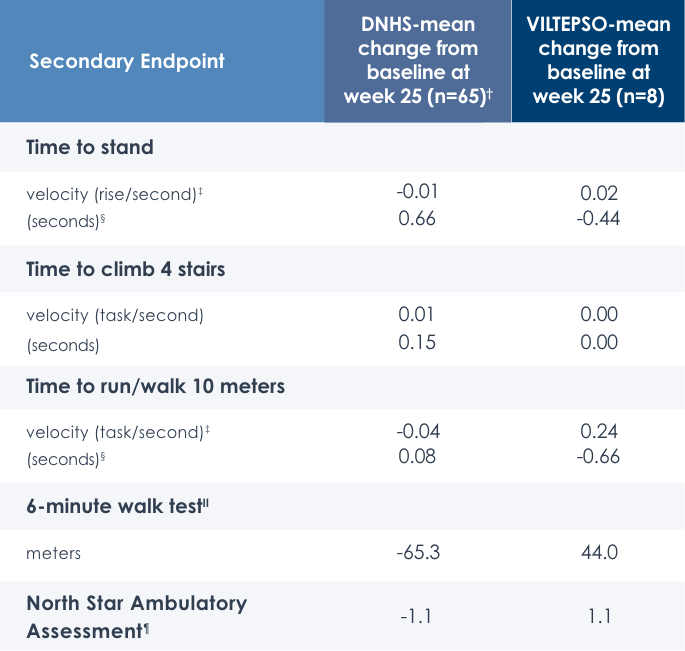
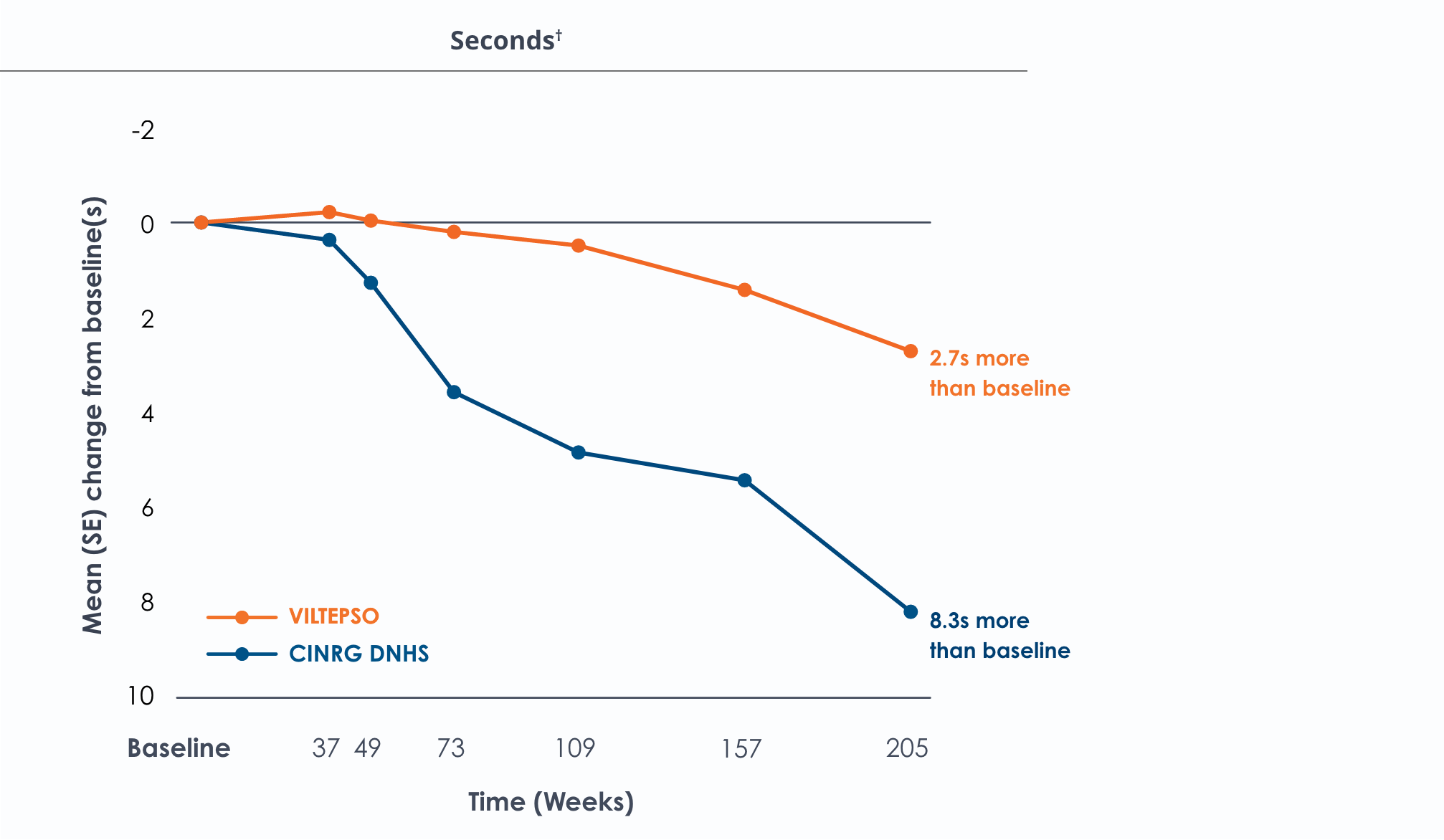
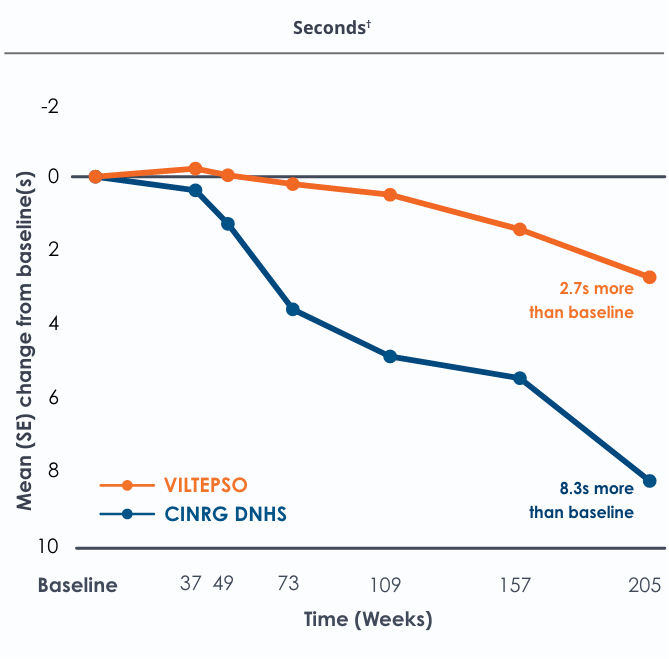
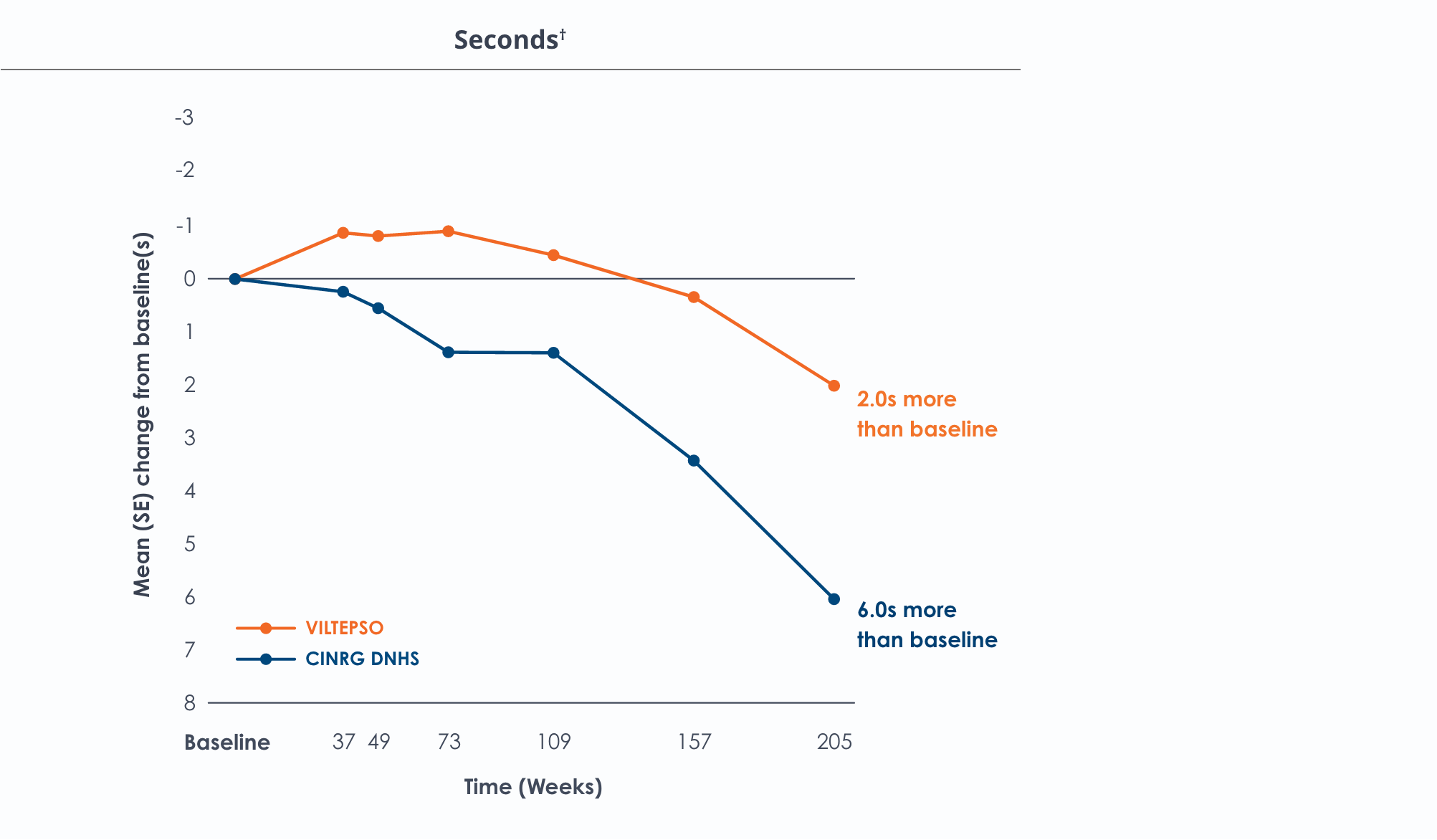
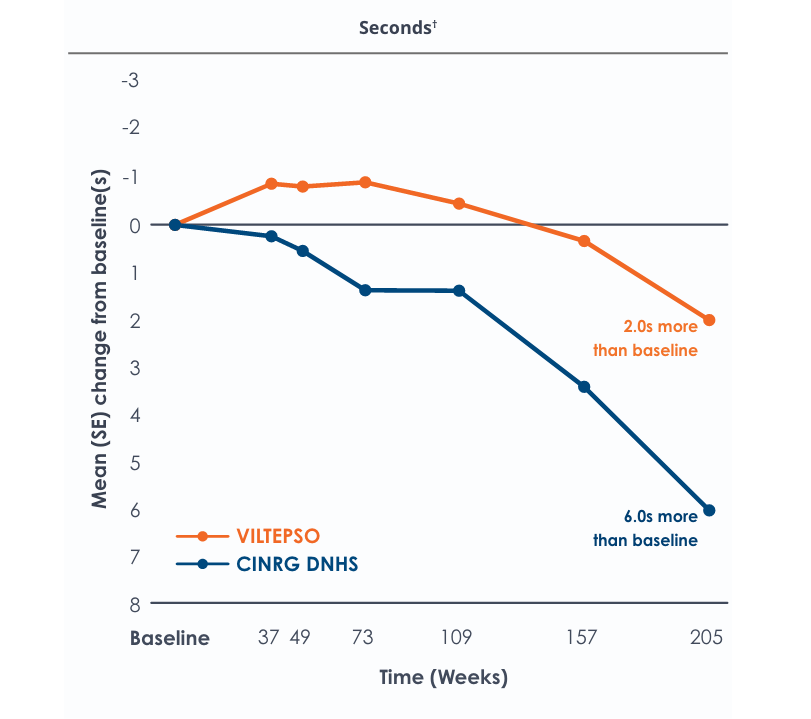
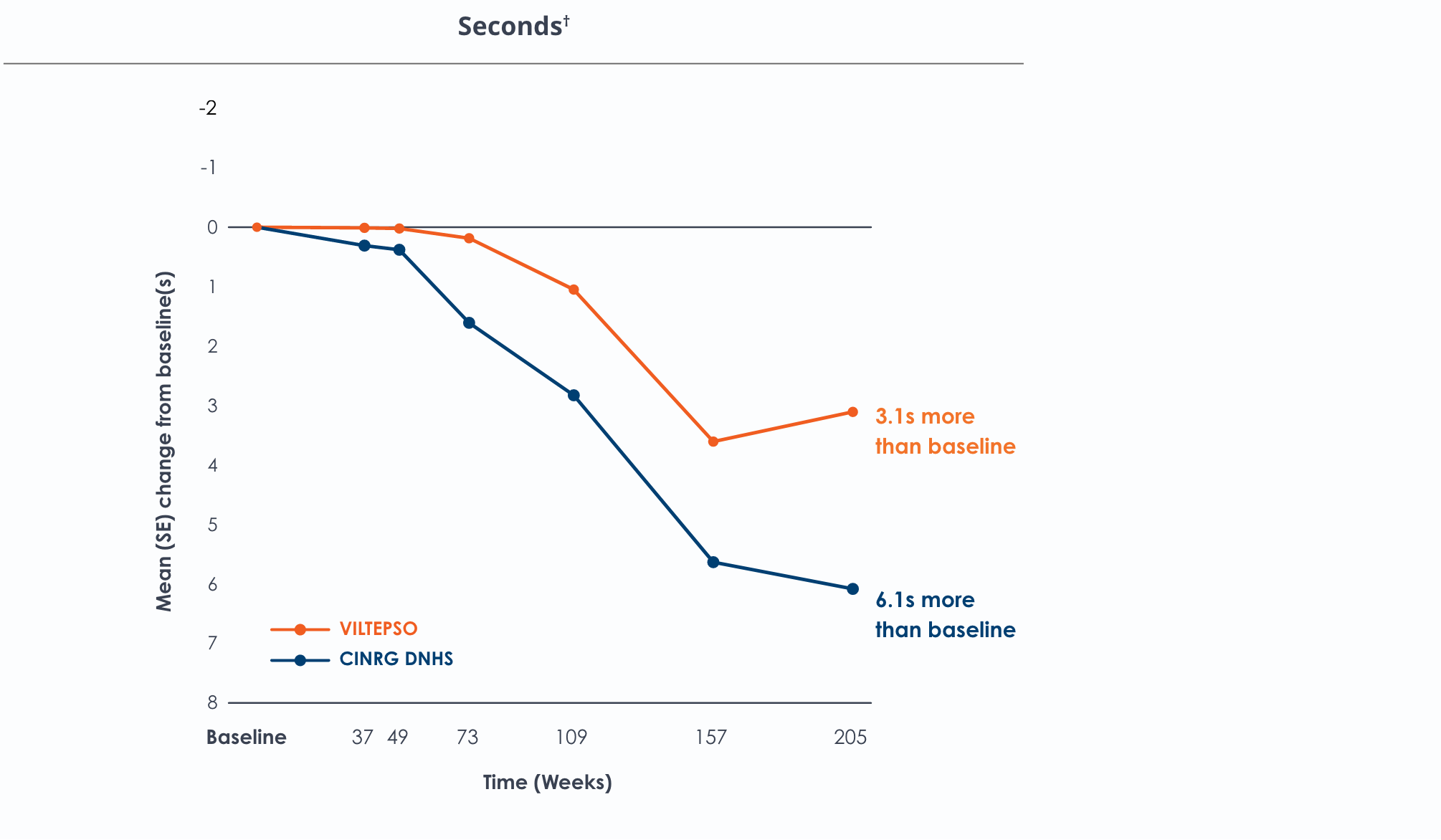
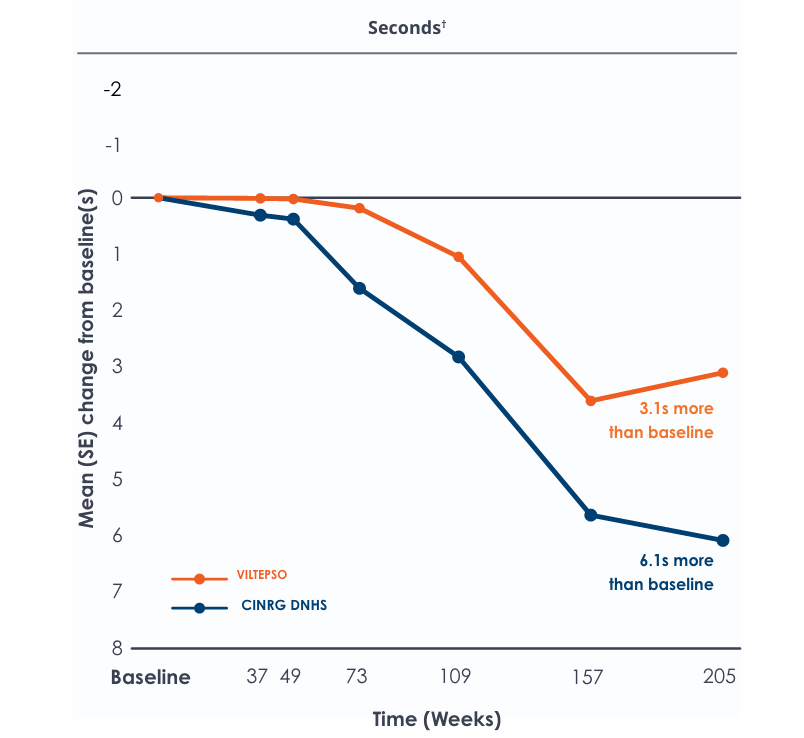
Motor Function Tests
Functional tests were compared to Duchenne natural history data as the control group rather than to placebo. Definitive conclusions should not be drawn. Functional data are not in the US Prescribing Information.