Impact on Dystrophin Levels
VILTEPSO significantly increased dystrophin production


88% (7 out of 8 people) showed increases of ~3% or higher, measured at week 25 of treatment.
These significant increases in dystrophin production with VILTEPSO were identified by a method called western blot and verified by a highly sensitive measuring technique known as mass spectrometry.
VILTEPSO increased dystrophin levels to
nearly 6% of normal


VILTEPSO was studied in 16 ambulatory (walking) boys ages 4 to less than 10 years who were receiving a stable dose of corticosteroids for at least 3 months.
In this graph, their average dystrophin levels at week 25 of VILTEPSO treatment are compared with their average dystrophin levels before treatment.
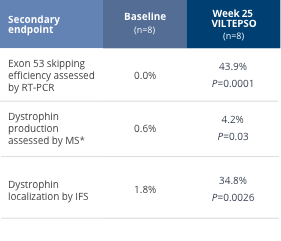
Secondary Endpoints
In the same clinical study, additional results
included evidence of dystrophin production and
timed motor function tests
Dystrophin Production Evidence

Motor Function Tests
Functional tests were compared to Duchenne natural history (DNHS) data as the control group rather than to placebo. Definitive conclusions should not be drawn. Functional data are not in the US Prescribing Information.


Safety
Safety profile evaluated in two 24-week clinical studies
Adverse reactions reported in ≥10% of people with DMD treated with VILTEPSO 80 mg/kg once weekly


No patients in the clinical trial discontinued treatment as a result of treatment-related Serious Adverse Events (SAEs)
MUSCLE FUNCTION DATA
An extended view of patients on VILTEPSO over 4 years
Functional tests were compared to Duchenne natural history data as the control group rather than to placebo.
Definitive conclusions should not be drawn. Functional data are not in the US Prescribing Information.
Time to stand over 4 years*
With VILTEPSO, the mean change from baseline at week 205 was 2.7 seconds and in the CINRG group the mean change from baseline at week 205 was 8.3 seconds.
Time to stand measures the amount of time it takes for a DMD patient to go from lying on their back to standing.
Seconds†


Time to run/walk 10 meters over 4 years*
With VILTEPSO, the mean change from baseline at week 205 was 2.0 seconds and in the CINRG group the mean change from baseline at week 205 was 6.0 seconds.
Time to run/walk 10 meters measures the amount of time it takes for a DMD patient to run or walk 10 meters.
Seconds†


Time to climb four stairs over 4 years*
With VILTEPSO, the mean change from baseline at week 205 was 3.1 seconds and in the CINRG group the mean change from baseline at week 205 was 6.1 seconds.
Time to climb four stairs measures the amount of time it takes for a DMD patient to climb four stairs.
Seconds†


†Negative time means less time; positive time means more time.
CINRG=Cooperative International Neuromuscular Research Group; DNHS=Duchenne Natural History Study.
FOUR-YEAR SAFETY DATA
Safety assessment for open-label,
four-year extension study data


AE=adverse event; TEAE=treatment-emergent AE; wk=week.
No patients discontinued the study as a result of
treatment-related Serious Adverse Events (SAEs)





Meet the VILTEPSO Ambassadors
Hear and read what real patients and their families have to say about life and treatment with VILTEPSO.
See Patient Stories


REMEMBER, A DOCTOR IS ALWAYS YOUR BEST RESOURCE FOR information about DMD and determining if VILTEPSO could be the right treatment option for you.
For more information about DMD, exon skipping, and VILTEPSO, you can download the Caregiver and Patient Guide
DOWNLOAD THE GUIDEFor suggested questions to ask your doctor about DMD, exon skipping, and VILTEPSO, you can download the Doctor Discussion Guide
DOWNLOAD THE GUIDE
833-NSSUPRT (833-677-8778)
Monday–Friday, 8 AM-8 PM ET
Discover NS Support and find links to helpful resources
NS Pharma is fully committed to providing ongoing support to DMD patients, their caregivers, and healthcare professionals.
Get Support